Article preview View full access options
Nature | Article
A new antibiotic kills pathogens without detectable resistance
- Losee L. Ling,1, n1
- Tanja Schneider,2, 3, n1
- Aaron J. Peoples,1,
- Amy L. Spoering,1,
- Ina Engels,2, 3,
- Brian P. Conlon,4,
- Anna Mueller,2, 3,
- Till F. Schäberle,3, 5,
- Dallas E. Hughes,1,
- Slava Epstein,6,
- Michael Jones,7,
- Linos Lazarides,7,
- Victoria A. Steadman,7,
- Douglas R. Cohen,1,
- Cintia R. Felix,1,
- K. Ashley Fetterman,1,
- William P. Millett,1,
- Anthony G. Nitti,1,
- Ashley M. Zullo,1,
- Chao Chen4,
- & Kim Lewis4,
- Journal name:
- Nature
- Year published:
- DOI:
- doi:10.1038/nature14098
- Received
- Accepted
- Published online
Abstract
Antibiotic resistance is spreading faster than the introduction of new compounds into clinical practice, causing a public health crisis. Most antibiotics were produced by screening soil microorganisms, but this limited resource of cultivable bacteria was overmined by the 1960s. Synthetic approaches to produce antibiotics have been unable to replace this platform. Uncultured bacteria make up approximately 99% of all species in external environments, and are an untapped source of new antibiotics. We developed several methods to grow uncultured organisms by cultivation in situ or by using specific growth factors. Here we report a new antibiotic that we term teixobactin, discovered in a screen of uncultured bacteria. Teixobactin inhibits cell wall synthesis by binding to a highly conserved motif of lipid II (precursor of peptidoglycan) and lipid III (precursor of cell wall teichoic acid). We did not obtain any mutants of Staphylococcus aureus or Mycobacterium tuberculosis resistant to teixobactin. The properties of this compound suggest a path towards developing antibiotics that are likely to avoid development of resistance.
At a glance
Figures
-
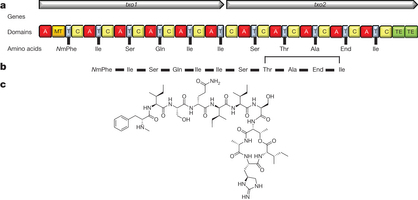
Figure 1: The structure of teixobactin and the predicted biosynthetic gene cluster. a, The two NRPS genes, the catalytic domains they encode, and the amino acids incorporated by the respective modules. Domains: A, adenylation; C, condensation; MT, methylation (of phenylalanine); T, thiolation (carrier); and TE, thioesterase (Ile-Thr ring closure). NmPhe, N-methylated phenylalanine. b, Schematic structure of teixobactin. The N-methylation of the first phenylalanine is catalysed by the methyltransferase domain in module 1. The ring closure between the last isoleucine and threonine is catalysed by the thioesterase domains during molecule off-loading, resulting in teixobactin. c, Teixobactin structure.
-
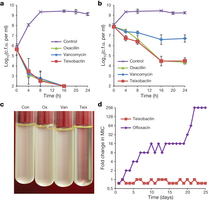
Figure 2: Time-dependent killing of pathogens by teixobactin. a, b, S. aureus were grown to early (a), and late (b) exponential phase and challenged with antibiotics. Data are representative of 3 independent experiments ± s.d. c, Teixobactin treatment resulted in lysis. The figure is representative of 3 independent experiments. d, Resistance acquisition during serial passaging in the presence of sub-MIC levels of antimicrobials. The y axis is the highest concentration the cells grew in during passaging. For ofloxacin, 256 × MIC was the highest concentration tested. The figure is representative of 3 independent experiments.
-
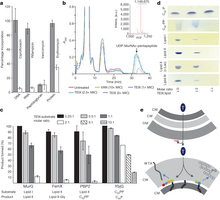
Figure 3: Teixobactin binds to cell wall precursors. a, Impact of teixobactin (TEIX) on macromolecular biosyntheses in S. aureus. Incorporation of 3H-thymidine (DNA), 3H-uridine (RNA), 3H-leucine (protein), and 3H-glucosamine (peptidoglycan) was determined in cells treated with teixobactin at 1 × MIC (grey bars). Ciprofloxacin (8 × MIC), rifampicin (4 × MIC), vancomycin (2 × MIC) and erythromycin (2 × MIC) were used as controls (white bars). Data are means of 4 independent experiments ± s.d. b, Intracellular accumulation of the cell wall precursor UDP-MurNAc-pentapeptide after treatment of S. aureus with teixobactin. Untreated and vancomycin (VAN)-treated (10 × MIC) cells were used as controls. UDP-MurNAc-pentapeptide was identified by mass spectrometry as indicated by the peak at m/z 1,149.675. The experiment is representative of 3 independent experiments. c, The effect of teixobactin on precursor consuming reactions. Experiments were performed in 3 biological replicates and data are presented as mean ± s.d. d, Complex formation of teixobactin with purified cell wall precursors. Binding of teixobactin is indicated by a reduction of the amount of lipid intermediates (visible on the thin-layer chromatogram). The figure is representative of two independent experiments. e, A model of teixobactin targeting and resistance. The teixobactin producer is a Gram-negative bacterium protected from this compound by exporting it across the outer membrane permeability barrier (upper panel). In target Gram-positive organisms lacking an outer membrane, the targets are readily accessible on the outside where teixobactin binds precursors of peptidoglycan (PG) and WTA. CM, cytoplasmic membrane; CW, cell wall; OM, outer membrane; T, teixobactin.
-

Figure 4: Teixobactin is efficacious in three mouse models of infection. a, Single dose treatment (i.v., 1 h post-infection, 6 mice per group) with teixobactin and vancomycin in septicemia protection model using MRSA. Survival is depicted 48 h after infection. b, Single dose (i.v., 2 h post-infection, 4 mice per group) treatment with teixobactin and vancomycin in neutropenic mouse thigh infection model using MRSA. For drug-treated animals, thigh colony-forming units (c.f.u.) were determined at 26 h post-infection. For controls, c.f.u. in thighs were determined at 2 h and 26 h post-infection. c, Two dose treatment, 5 mice per group, with teixobactin (i.v., 24 h and 36 h post-infection) and single dose treatment with amoxicillin (subcutaneous, 24 h post-infection) in immunocompetent lung infection model using S. pneumoniae. Lung c.f.u. were determined at 48 h post-infection. The c.f.u. from each mouse are plotted as individual points and error bars represent the deviation within an experimental group. *P < 0.05, ***P < 0.001 (determined by non-parametric log-rank test).
-
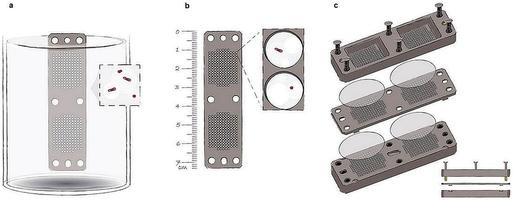
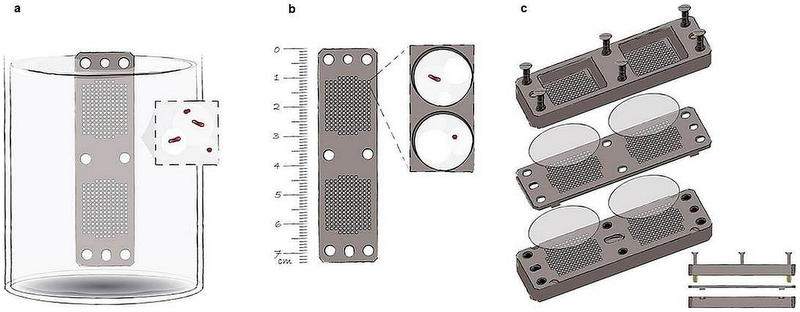
Extended Data Fig. 1: The iChip. a–c, The iChip (a) consists of a central plate (b) which houses growing microorganisms, semi-permeable membranes on each side of the plate, which separate the plate from the environment, and two supporting side panels (c). The central plate and side panels have multiple matching through-holes. When the central plate is dipped into suspension of cells in molten agar, the through-holes capture small volumes of this suspension, which solidify in the form of small agar plugs. Alternatively, molten agar can be dispensed into the chambers. The membranes are attached and the iChip is then placed in soil from which the sample originated.
-
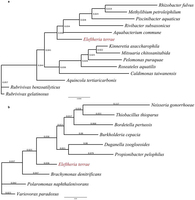
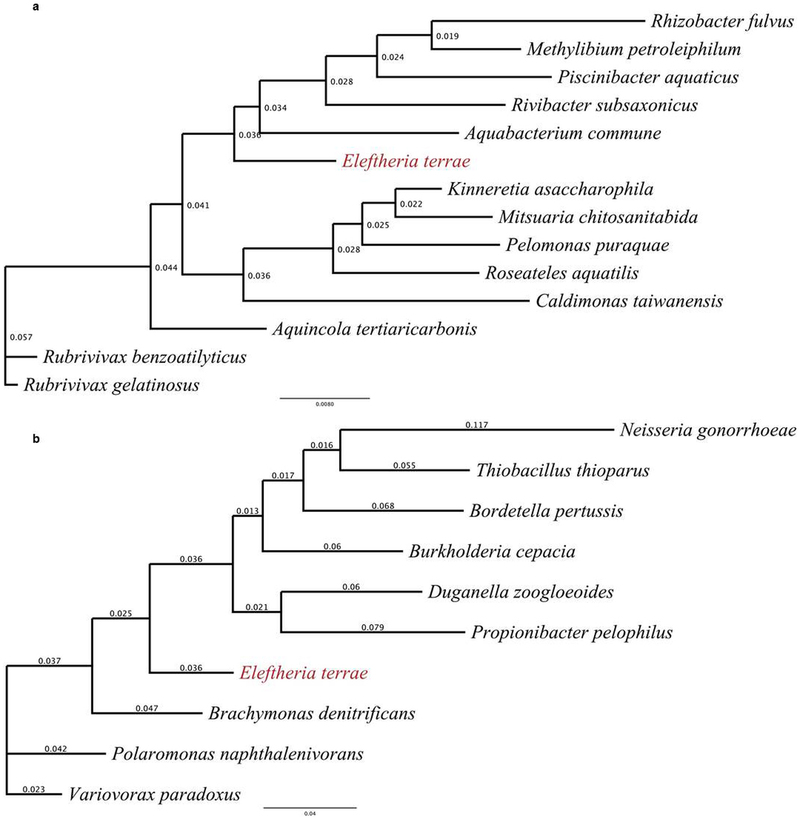
Extended Data Fig. 2: 16S rRNA gene phylogeny of Eleftheria terrae. a, The phylogenetic position of E. terrae within the class β-proteobacteria. The 16S rRNA gene sequences were downloaded from Entrez at NCBI using accession numbers retrieved from peer-reviewed publications. b, The phylogenetic position of E. terrae among its closest known relatives. The sequences were downloaded from NCBI using accession numbers retrieved from the RDP Classifier Database. For both trees, multiple sequence alignments (MSA) were constructed using ClustalW2, implementing a default Cost Matrix, the Neighbour-Joining (NJ) clustering algorithm, as well as optimized gap penalties. Resulting alignments were manually curated and phylogenetic trees were constructed leveraging PhyML 3.0 with a TN93 substitution model and 500 Bootstrap iterations of branch support. Topology search optimization was conducted using the Subtree–Pruning–Regrafting (SPR) algorithm with an estimated Transition–Transversion ratio and gamma distribution parameters as well as fixed proportions of invariable sites.
-

Extended Data Fig. 3: NMR assignment of teixobactin. a, 13C-NMR of teixobactin (125 mHz, δ in p.p.m.). b, Structure of teixobactin with the NMR assignments.
-

Extended Data Fig. 4: NMR spectra of teixobactin. a, 13C NMR spectrum of teixobactin. b, 1H NMR spectrum. c, HMBC NMR spectrum. d, HSQC NMR spectrum. e, COSY NMR spectrum.
-
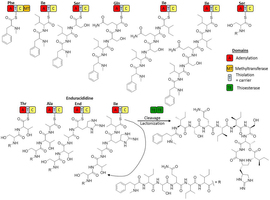
Extended Data Fig. 5: Hypothetical biosynthesis pathway of teixobactin. The eleven modules of the non-ribosomal peptide synthetases Txo1 and Txo2 are depicted with the growing chain attached. Each module is responsible for the incorporation of one specific amino acid in the nascent peptide chain. The N-methylation of the first amino acid phenylalanine is catalysed by the methyltransferase domain in module 1. The ring closure (marked by a dashed arrow) between the last isoleucine and threonine is catalysed by the thioesterase domains during molecule off-loading, resulting in teixobactin.
-
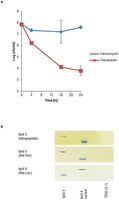
Extended Data Fig. 6: Teixobactin activity against vancomycin-resistant strains. a, Vancomycin intermediate S. aureus (VISA) were grown to late exponential phase and challenged with vancomycin or teixobactin. Cell numbers were determined by plating for colony counts. Data are representative of 3 independent experiments ± s.d. b, Complex formation of teixobactin with cell wall precursor variants as formed by vancomycin-resistant strains. Purified lipid intermediates with altered stem peptides were incubated with teixobactin at a molar ratio of 2:1 (TEIX:lipid II variant). Reaction mixtures were extracted with BuOH/PyrAc and binding of teixobactin to lipid II variants is indicated by its absence on the thin-layer chromatogram. Migration behaviour of unmodified lipid II is used for comparison. The figure is representative of 3 independent experiments.
-
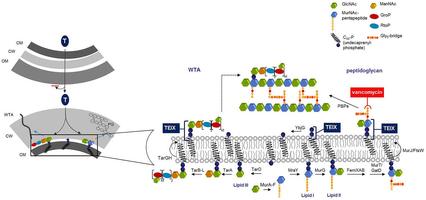
Extended Data Fig. 7: Model for the mechanism of action of teixobactin. Inhibition of cell wall synthesis by teixobactin. Lipid II, precursor of peptidoglycan, is synthesized in the cytoplasm and flipped to the surface of the inner membrane by MurJ48 or FtsW49. Lipid III, a precursor of wall teichoic acid (WTA), is similarly formed inside the cell and WTA lipid-bound precursors are translocated across the cytoplasmic membrane by the ABC-transporter TarGH50. Teixobactin (TEIX) forms a stoichiometric complex with cell wall precursors, lipid II and lipid III. Abduction of these building blocks simultaneously interrupts peptidoglycan (right), WTA (left) biosynthesis as well as precursor recycling. Binding to multiple targets within the cell wall pathways obstructs the formation of a functional cell envelope. Left panel, teixobactin targeting and resistance. The producer of teixobactin is a Gram-negative bacterium which is protected from this compound by exporting it outside of its outer membrane permeability barrier. The target Gram-positive organisms do not have an outer membrane. CM, cytoplasmic membrane; CW, cell wall; OM, outer membrane; LTA, lipoteichoic acid; WTA, wall teichoic acid.
-
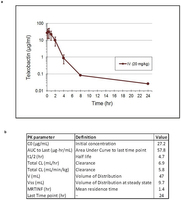
Extended Data Fig. 8: Pharmacokinetic analysis of teixobactin. a, The mean plasma concentrations of teixobactin after a single i.v. injection of 20 mg per kg teixobactin (3 mice per time point). Data are the mean of plasma concentration, and error bars represent the standard deviation from 3 animals in each time point. b, Pharmacokinetic parameters of teixobactin calculated with a non-compartmental analysis model based on WinNonlin.
Read the full article
Additional access options:
- British Library Document Supply Centre
- You can also request this document from your local library through inter-library loan services.
Accession codes
Primary accessions
GenBank/EMBL/DDBJ
References
- Fleming, A. On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. influenzae. Br. J. Exp. Pathol. 10, 226–236 (1929)
- Kardos, N. & Demain, A. L. Penicillin: the medicine with the greatest impact on therapeutic outcomes. Appl. Microbiol. Biotechnol. 92, 677–687 (2011)
- Schatz, A., Bugie, E. & Waksman, S. A. Streptomycin, a substance exhibiting antibiotic activity against gram-positive and gram-negative bacteria. Proc. Soc. Exp. Biol. Med. 55, 66–69 (1944)
- Spellberg, B. & Shlaes, D. Prioritized current unmet needs for antibacterial therapies. Clin. Pharmacol. Ther. 96, 151–153 (2014)
- Bush, K. et al. Tackling antibiotic resistance. Nature Rev. Microbiol. 9, 894–896 (2011)
- Payne, D. J., Gwynn, M. N., Holmes, D. J. & Pompliano, D. L. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nature Rev. Drug Discov. 6, 29–40 (2007)
- Lewis, K. Antibiotics: Recover the lost art of drug discovery. Nature 485, 439–440 (2012)
- Lewis, K. Platforms for antibiotic discovery. Nature Rev. Drug Discov. 12, 371–387 (2013)
- Kaeberlein, T., Lewis, K. & Epstein, S. S. Isolating “uncultivable” microorganisms in pure culture in a simulated natural environment. Science 296, 1127–1129 (2002)
- Nichols, D. et al. Use of ichip for high-throughput in situ cultivation of “uncultivable” microbial species. Appl. Environ. Microbiol. 76, 2445–2450 (2010)
- D’Onofrio, A. et al. Siderophores from neighboring organisms promote the growth of uncultured bacteria. Chem. Biol. 17, 254–264 (2010)
- Gavrish, E. et al. Lassomycin, a ribosomally synthesized cyclic peptide, kills Mycobacterium tuberculosis by targeting the ATP-dependent protease ClpC1P1P2. Chem. Biol. 21, 509–518 (2014)
- Wilson, M. C. et al. An environmental bacterial taxon with a large and distinct metabolic repertoire. Nature 506, 58–62 (2014)
- Nichols, D. et al. Short peptide induces an “uncultivable” microorganism to grow in vitro. Appl. Environ. Microbiol. 74, 4889–4897 (2008)
- Sakoulas, G. et al. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J. Clin. Microbiol. 42, 2398–2402 (2004)
- Kollef, M. H. Limitations of vancomycin in the management of resistant staphylococcal infections. Clin. Infect. Dis. 45 (Suppl 3). S191–S195 (2007)
- Arthur, M., Depardieu, F., Reynolds, P. & Courvalin, P. Quantitative analysis of the metabolism of soluble cytoplasmic peptidoglycan precursors of glycopeptide-resistant enterococci. Mol. Microbiol. 21, 33–44 (1996)
- Bugg, T. D. et al. Molecular basis for vancomycin resistance in Enterococcus faecium BM4147: biosynthesis of a depsipeptide peptidoglycan precursor by vancomycin resistance proteins VanH and VanA. Biochemistry 30, 10408–10415 (1991)
- Marshall, C. G., Broadhead, G., Leskiw, B. K. & Wright, G. D. d-Ala-d-Ala ligases from glycopeptide antibiotic-producing organisms are highly homologous to the enterococcal vancomycin-resistance ligases VanA and VanB. Proc. Natl Acad. Sci. USA 94, 6480–6483 (1997)
- D’Elia, M. A. et al. Lesions in teichoic acid biosynthesis in Staphylococcus aureus lead to a lethal gain of function in the otherwise dispensable pathway. J. Bacteriol. 188, 4183–4189 (2006)
- Bierbaum, G. & Sahl, H. G. Induction of autolysis of staphylococci by the basic peptide antibiotics Pep 5 and nisin and their influence on the activity of autolytic enzymes. Arch. Microbiol. 141, 249–254 (1985)
- O’Riordan, K. & Lee, J. C. Staphylococcus aureus capsular polysaccharides. Clin. Microbiol. Rev. 17, 218–234 (2004)
- Xayarath, B. & Yother, J. Mutations blocking side chain assembly, polymerization, or transport of a Wzy-dependent Streptococcus pneumoniae capsule are lethal in the absence of suppressor mutations and can affect polymer transfer to the cell wall. J. Bacteriol. 189, 3369–3381 (2007)
- Degen, D. et al. Transcription inhibition by the depsipeptide antibiotic salinamide A. eLife 3, e02451 (2014)
- Doroghazi, J. R. et al. A roadmap for natural product discovery based on large-scale genomics and metabolomics. Nature Chem. Biol. 10, 963–968 (2014)
- Forsberg, K. J. et al. Bacterial phylogeny structures soil resistomes across habitats. Nature 509, 612–616 (2014)
- Conlon, B. P. et al. Activated ClpP kills persisters and eradicates a chronic biofilm infection. Nature 503, 365–370 (2013)
- Schneider, T. & Sahl, H. G. An oldie but a goodie—cell wall biosynthesis as antibiotic target pathway. Int. J. Med. Microbiol. 300, 161–169 (2010)
- Leclercq, R., Derlot, E., Duval, J. & Courvalin, P. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N. Engl. J. Med. 319, 157–161 (1988)
- Wiedemann, I. et al. Specific binding of nisin to the peptidoglycan precursor lipid II combines pore formation and inhibition of cell wall biosynthesis for potent antibiotic activity. J. Biol. Chem. 276, 1772–1779 (2001)
- Hasper, H. E. et al. An alternative bactericidal mechanism of action for lantibiotic peptides that target lipid II. Science 313, 1636–1637 (2006)
- Schneider, T. et al. Plectasin, a fungal defensin, targets the bacterial cell wall precursor Lipid II. Science 328, 1168–1172 (2010)
- Baker, G. C., Smith, J. J. & Cowan, D. A. Review and re-analysis of domain-specific 16S primers. J. Microbiol. Methods 55, 541–555 (2003)
- Aziz, R. K. et al. The RAST Server: rapid annotations using subsystems technology. BMC Genomics 9, 75 (2008)
- Auch, A. F., Klenk, H. P. & Goker, M. Standard operating procedure for calculating genome-to-genome distances based on high-scoring segment pairs. Stand. Genomic Sci. 2, 142–148 (2010)
- Auch, A. F., von Jan, M., Klenk, H. P. & Goker, M. Digital DNA–DNA hybridization for microbial species delineation by means of genome-to-genome sequence comparison. Stand. Genomic Sci. 2, 117–134 (2010)
- Meier-Kolthoff, J. P., Auch, A. F., Klenk, H. P. & Goker, M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics 14, 60 (2013)
- Röttig, M. et al. NRPSpredictor2–a web server for predicting NRPS adenylation domain specificity. Nucleic Acids Res. 39, W362–W367 (2011)
- Arhin, F. F. et al. Effect of polysorbate 80 on oritavancin binding to plastic surfaces: implications for susceptibility testing. Antimicrob. Agents Chemother. 52, 1597–1603 (2008)
- Bogdanovich, T., Ednie, L. M., Shapiro, S. & Appelbaum, P. C. Antistaphylococcal activity of ceftobiprole, a new broad-spectrum cephalosporin. Antimicrob. Agents Chemother. 49, 4210–4219 (2005)
- Metzler, K., Drlica, K. & Blondeau, J. M. Minimal inhibitory and mutant prevention concentrations of azithromycin, clarithromycin and erythromycin for clinical isolates of Streptococcus pneumoniae. J. Antimicrob. Chemother. 68, 631–635 (2013)
- Schneider, T. et al. The lipopeptide antibiotic Friulimicin B inhibits cell wall biosynthesis through complex formation with bactoprenol phosphate. Antimicrob. Agents Chemother. 53, 1610–1618 (2009)
- Brötz, H., Bierbaum, G., Reynolds, P. E. & Sahl, H. G. The lantibiotic mersacidin inhibits peptidoglycan biosynthesis at the level of transglycosylation. Eur. J. Biochem. 246, 193–199 (1997)
- Müller, A., Ulm, H., Reder-Christ, K., Sahl, H. G. & Schneider, T. Interaction of type A lantibiotics with undecaprenol-bound cell envelope precursors. Microb. Drug Resist. 18, 261–270 (2012)
- Schneider, T. et al. In vitro assembly of a complete, pentaglycine interpeptide bridge containing cell wall precursor (lipid II-Gly5) of Staphylococcus aureus. Mol. Microbiol. 53, 675–685 (2004)
- El Ghachi, M., Derbise, A., Bouhss, A. & Mengin–Lecreulx, D. Identification of multiple genes encoding membrane proteins with undecaprenyl pyrophosphate phosphatase (UppP) activity in Escherichia coli. J. Biol. Chem. 280, 18689–18695 (2005)
- El Ghachi, M., Bouhss, A., Blanot, D. & Mengin–Lecreulx, D. The bacA gene of Escherichia coli encodes an undecaprenyl pyrophosphate phosphatase activity. J. Biol. Chem. 279, 30106–30113 (2004)
- Sham, L. T. et al. Bacterial cell wall. MurJ is the flippase of lipid-linked precursors for peptidoglycan biogenesis. Science 345, 220–222 (2014)
- Mohammadi, T. et al. Identification of FtsW as a transporter of lipid-linked cell wall precursors across the membrane. EMBO J. 30, 1425–1432 (2011)
- Lazarevic, V. & Karamata, D. The tagGH operon of Bacillus subtilis 168 encodes a two-component ABC transporter involved in the metabolism of two wall teichoic acids. Mol. Microbiol. 16, 345–355 (1995)
Author information
Author footnotes
These authors contributed equally to this work.
- Losee L. Ling &
- Tanja Schneider
Affiliations
-
NovoBiotic Pharmaceuticals, Cambridge, Massachusetts 02138, USA
- Losee L. Ling,
- Aaron J. Peoples,
- Amy L. Spoering,
- Dallas E. Hughes,
- Douglas R. Cohen,
- Cintia R. Felix,
- K. Ashley Fetterman,
- William P. Millett,
- Anthony G. Nitti &
- Ashley M. Zullo
-
Institute of Medical Microbiology, Immunology and Parasitology—Pharmaceutical Microbiology Section, University of Bonn, Bonn 53115, Germany
- Tanja Schneider,
- Ina Engels &
- Anna Mueller
-
German Centre for Infection Research (DZIF), Partner Site Bonn-Cologne, 53115 Bonn, Germany
- Tanja Schneider,
- Ina Engels,
- Anna Mueller &
- Till F. Schäberle
-
Antimicrobial Discovery Center, Northeastern University, Department of Biology, Boston, Massachusetts 02115, USA
- Brian P. Conlon,
- Chao Chen &
- Kim Lewis
-
Institute for Pharmaceutical Biology, University of Bonn, Bonn 53115, Germany
- Till F. Schäberle
-
Department of Biology, Northeastern University, Boston, Massachusetts 02115, USA
- Slava Epstein
-
Selcia, Ongar, Essex CM5 0GS, UK
- Michael Jones,
- Linos Lazarides &
- Victoria A. Steadman
Contributions
K.L. and T.S. designed the study, analysed results, and wrote the paper. L.L.L. designed the study and analysed results. A.J.P. designed the study, performed compound isolation and structure determination and analysed data. B.P.C. designed the study, performed susceptibility experiments and wrote the paper. D.E.H. oversaw preclinical work including designing studies and analysing data. S.E. designed cultivation experiments and analysed data. M.J., L.L. and V.A.S. designed and performed experiments on structure determination and analysed data. I.E. and A.M. designed and performed experiments on mechanism of action. A.L.S., D.R.C., C.R.F., K.A.F., W.P.M., A.G.N., A.M.Z. and C.C. performed experiments on compound production, isolation, susceptibility testing and data analysis. T.F.S. identified the biosynthetic cluster.
Competing financial interests
The following authors, L. L. Ling, A. J. Peoples, A. L. Spoering, D. E. Hughes, D. R. Cohen, C. R. Felix, K. A. Fetterman, W. P. Millett, A. G. Nitti, A. M. Zullo, K. Lewis, and S. Epstein, declare competing financial interests as they are employees and consultants of NovoBiotic Pharmaceuticals.
The biosynthetic gene cluster for teixobactin has been deposited with GenBank under accession number KP006601.
Author details
Losee L. Ling
Search for this author in:
Tanja Schneider
Search for this author in:
Aaron J. Peoples
Search for this author in:
Amy L. Spoering
Search for this author in:
Ina Engels
Search for this author in:
Brian P. Conlon
Search for this author in:
Anna Mueller
Search for this author in:
Till F. Schäberle
Search for this author in:
Dallas E. Hughes
Search for this author in:
Slava Epstein
Search for this author in:
Michael Jones
Search for this author in:
Linos Lazarides
Search for this author in:
Victoria A. Steadman
Search for this author in:
Douglas R. Cohen
Search for this author in:
Cintia R. Felix
Search for this author in:
K. Ashley Fetterman
Search for this author in:
William P. Millett
Search for this author in:
Anthony G. Nitti
Search for this author in:
Ashley M. Zullo
Search for this author in:
Chao Chen
Search for this author in:
Kim Lewis
Search for this author in:
Extended data figures and tables
Extended Data Figures
- Extended Data Figure 1: The iChip. (183 KB)
a–c, The iChip (a) consists of a central plate (b) which houses growing microorganisms, semi-permeable membranes on each side of the plate, which separate the plate from the environment, and two supporting side panels (c). The central plate and side panels have multiple matching through-holes. When the central plate is dipped into suspension of cells in molten agar, the through-holes capture small volumes of this suspension, which solidify in the form of small agar plugs. Alternatively, molten agar can be dispensed into the chambers. The membranes are attached and the iChip is then placed in soil from which the sample originated.
- Extended Data Figure 2: 16S rRNA gene phylogeny of Eleftheria terrae. (191 KB)
a, The phylogenetic position of E. terrae within the class β-proteobacteria. The 16S rRNA gene sequences were downloaded from Entrez at NCBI using accession numbers retrieved from peer-reviewed publications. b, The phylogenetic position of E. terrae among its closest known relatives. The sequences were downloaded from NCBI using accession numbers retrieved from the RDP Classifier Database. For both trees, multiple sequence alignments (MSA) were constructed using ClustalW2, implementing a default Cost Matrix, the Neighbour-Joining (NJ) clustering algorithm, as well as optimized gap penalties. Resulting alignments were manually curated and phylogenetic trees were constructed leveraging PhyML 3.0 with a TN93 substitution model and 500 Bootstrap iterations of branch support. Topology search optimization was conducted using the Subtree–Pruning–Regrafting (SPR) algorithm with an estimated Transition–Transversion ratio and gamma distribution parameters as well as fixed proportions of invariable sites.
- Extended Data Figure 3: NMR assignment of teixobactin. (371 KB)
a, 13C-NMR of teixobactin (125 mHz, δ in p.p.m.). b, Structure of teixobactin with the NMR assignments.
- Extended Data Figure 4: NMR spectra of teixobactin. (224 KB)
a, 13C NMR spectrum of teixobactin. b, 1H NMR spectrum. c, HMBC NMR spectrum. d, HSQC NMR spectrum. e, COSY NMR spectrum.
- Extended Data Figure 5: Hypothetical biosynthesis pathway of teixobactin. (220 KB)
The eleven modules of the non-ribosomal peptide synthetases Txo1 and Txo2 are depicted with the growing chain attached. Each module is responsible for the incorporation of one specific amino acid in the nascent peptide chain. The N-methylation of the first amino acid phenylalanine is catalysed by the methyltransferase domain in module 1. The ring closure (marked by a dashed arrow) between the last isoleucine and threonine is catalysed by the thioesterase domains during molecule off-loading, resulting in teixobactin.
- Extended Data Figure 6: Teixobactin activity against vancomycin-resistant strains. (267 KB)
a, Vancomycin intermediate S. aureus (VISA) were grown to late exponential phase and challenged with vancomycin or teixobactin. Cell numbers were determined by plating for colony counts. Data are representative of 3 independent experiments ± s.d. b, Complex formation of teixobactin with cell wall precursor variants as formed by vancomycin-resistant strains. Purified lipid intermediates with altered stem peptides were incubated with teixobactin at a molar ratio of 2:1 (TEIX:lipid II variant). Reaction mixtures were extracted with BuOH/PyrAc and binding of teixobactin to lipid II variants is indicated by its absence on the thin-layer chromatogram. Migration behaviour of unmodified lipid II is used for comparison. The figure is representative of 3 independent experiments.
- Extended Data Figure 7: Model for the mechanism of action of teixobactin. (182 KB)
Inhibition of cell wall synthesis by teixobactin. Lipid II, precursor of peptidoglycan, is synthesized in the cytoplasm and flipped to the surface of the inner membrane by MurJ48 or FtsW49. Lipid III, a precursor of wall teichoic acid (WTA), is similarly formed inside the cell and WTA lipid-bound precursors are translocated across the cytoplasmic membrane by the ABC-transporter TarGH50. Teixobactin (TEIX) forms a stoichiometric complex with cell wall precursors, lipid II and lipid III. Abduction of these building blocks simultaneously interrupts peptidoglycan (right), WTA (left) biosynthesis as well as precursor recycling. Binding to multiple targets within the cell wall pathways obstructs the formation of a functional cell envelope. Left panel, teixobactin targeting and resistance. The producer of teixobactin is a Gram-negative bacterium which is protected from this compound by exporting it outside of its outer membrane permeability barrier. The target Gram-positive organisms do not have an outer membrane. CM, cytoplasmic membrane; CW, cell wall; OM, outer membrane; LTA, lipoteichoic acid; WTA, wall teichoic acid.
- Extended Data Figure 8: Pharmacokinetic analysis of teixobactin. (239 KB)
a, The mean plasma concentrations of teixobactin after a single i.v. injection of 20 mg per kg teixobactin (3 mice per time point). Data are the mean of plasma concentration, and error bars represent the standard deviation from 3 animals in each time point. b, Pharmacokinetic parameters of teixobactin calculated with a non-compartmental analysis model based on WinNonlin.
Extended Data Tables
Supplementary information
PDF files
- Supplementary Information (187 KB)
This file contains a Supplementary Discussion.
Additional data
-
Extended Data Figure 1: The iChip.
 Hover over figure to zoom
Hover over figure to zooma–c, The iChip (a) consists of a central plate (b) which houses growing microorganisms, semi-permeable membranes on each side of the plate, which separate the plate from the environment, and two supporting side panels (c). The central plate and side panels have multiple matching through-holes. When the central plate is dipped into suspension of cells in molten agar, the through-holes capture small volumes of this suspension, which solidify in the form of small agar plugs. Alternatively, molten agar can be dispensed into the chambers. The membranes are attached and the iChip is then placed in soil from which the sample originated.
-
Extended Data Figure 2: 16S rRNA gene phylogeny of Eleftheria terrae.
 Hover over figure to zoom
Hover over figure to zooma, The phylogenetic position of E. terrae within the class β-proteobacteria. The 16S rRNA gene sequences were downloaded from Entrez at NCBI using accession numbers retrieved from peer-reviewed publications. b, The phylogenetic position of E. terrae among its closest known relatives. The sequences were downloaded from NCBI using accession numbers retrieved from the RDP Classifier Database. For both trees, multiple sequence alignments (MSA) were constructed using ClustalW2, implementing a default Cost Matrix, the Neighbour-Joining (NJ) clustering algorithm, as well as optimized gap penalties. Resulting alignments were manually curated and phylogenetic trees were constructed leveraging PhyML 3.0 with a TN93 substitution model and 500 Bootstrap iterations of branch support. Topology search optimization was conducted using the Subtree–Pruning–Regrafting (SPR) algorithm with an estimated Transition–Transversion ratio and gamma distribution parameters as well as fixed proportions of invariable sites.
-
Extended Data Figure 3: NMR assignment of teixobactin.
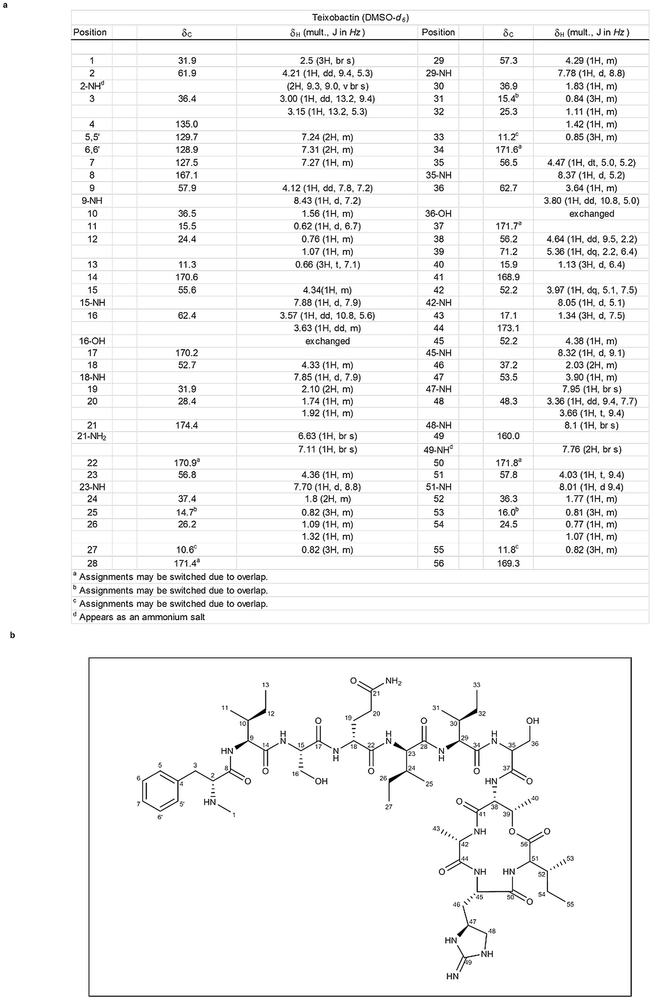 Hover over figure to zoom
Hover over figure to zooma, 13C-NMR of teixobactin (125 mHz, δ in p.p.m.). b, Structure of teixobactin with the NMR assignments.
-
Extended Data Figure 4: NMR spectra of teixobactin.
 Hover over figure to zoom
Hover over figure to zooma, 13C NMR spectrum of teixobactin. b, 1H NMR spectrum. c, HMBC NMR spectrum. d, HSQC NMR spectrum. e, COSY NMR spectrum.
-
Extended Data Figure 5: Hypothetical biosynthesis pathway of teixobactin.
 Hover over figure to zoom
Hover over figure to zoomThe eleven modules of the non-ribosomal peptide synthetases Txo1 and Txo2 are depicted with the growing chain attached. Each module is responsible for the incorporation of one specific amino acid in the nascent peptide chain. The N-methylation of the first amino acid phenylalanine is catalysed by the methyltransferase domain in module 1. The ring closure (marked by a dashed arrow) between the last isoleucine and threonine is catalysed by the thioesterase domains during molecule off-loading, resulting in teixobactin.
-
Extended Data Figure 6: Teixobactin activity against vancomycin-resistant strains.
 Hover over figure to zoom
Hover over figure to zooma, Vancomycin intermediate S. aureus (VISA) were grown to late exponential phase and challenged with vancomycin or teixobactin. Cell numbers were determined by plating for colony counts. Data are representative of 3 independent experiments ± s.d. b, Complex formation of teixobactin with cell wall precursor variants as formed by vancomycin-resistant strains. Purified lipid intermediates with altered stem peptides were incubated with teixobactin at a molar ratio of 2:1 (TEIX:lipid II variant). Reaction mixtures were extracted with BuOH/PyrAc and binding of teixobactin to lipid II variants is indicated by its absence on the thin-layer chromatogram. Migration behaviour of unmodified lipid II is used for comparison. The figure is representative of 3 independent experiments.
-
Extended Data Figure 7: Model for the mechanism of action of teixobactin.
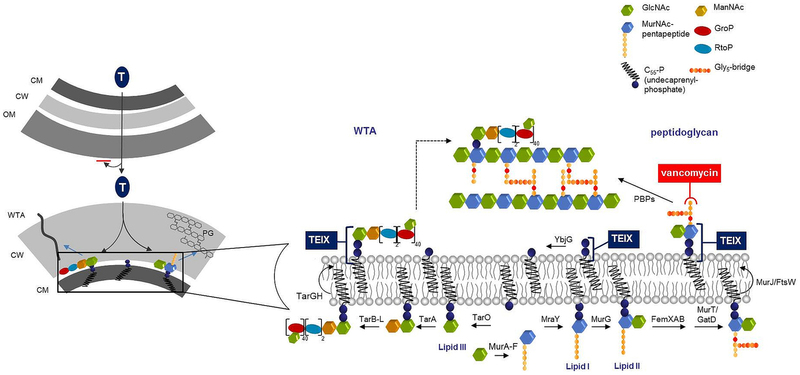 Hover over figure to zoom
Hover over figure to zoomInhibition of cell wall synthesis by teixobactin. Lipid II, precursor of peptidoglycan, is synthesized in the cytoplasm and flipped to the surface of the inner membrane by MurJ48 or FtsW49. Lipid III, a precursor of wall teichoic acid (WTA), is similarly formed inside the cell and WTA lipid-bound precursors are translocated across the cytoplasmic membrane by the ABC-transporter TarGH50. Teixobactin (TEIX) forms a stoichiometric complex with cell wall precursors, lipid II and lipid III. Abduction of these building blocks simultaneously interrupts peptidoglycan (right), WTA (left) biosynthesis as well as precursor recycling. Binding to multiple targets within the cell wall pathways obstructs the formation of a functional cell envelope. Left panel, teixobactin targeting and resistance. The producer of teixobactin is a Gram-negative bacterium which is protected from this compound by exporting it outside of its outer membrane permeability barrier. The target Gram-positive organisms do not have an outer membrane. CM, cytoplasmic membrane; CW, cell wall; OM, outer membrane; LTA, lipoteichoic acid; WTA, wall teichoic acid.
-
Extended Data Figure 8: Pharmacokinetic analysis of teixobactin.
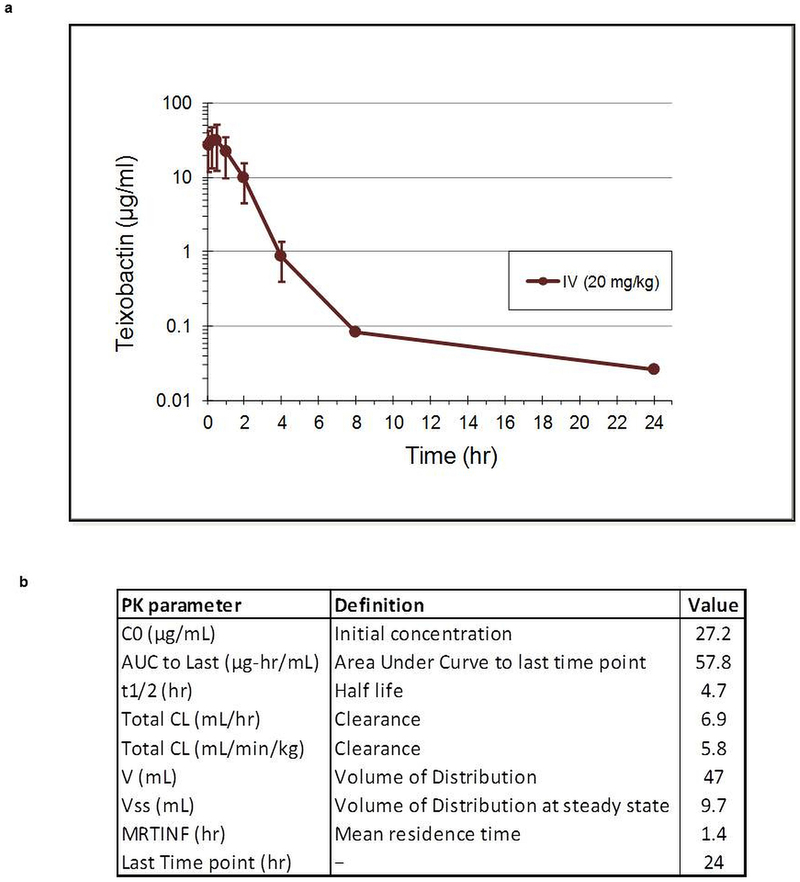 Hover over figure to zoom
Hover over figure to zooma, The mean plasma concentrations of teixobactin after a single i.v. injection of 20 mg per kg teixobactin (3 mice per time point). Data are the mean of plasma concentration, and error bars represent the standard deviation from 3 animals in each time point. b, Pharmacokinetic parameters of teixobactin calculated with a non-compartmental analysis model based on WinNonlin.
-
Extended Data Table 1: Antibacterial spectrum of teixobactin
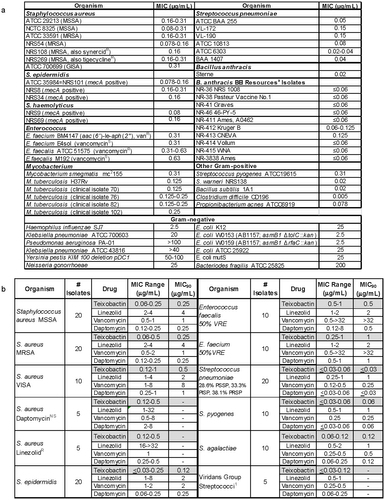 Hover over figure to zoom
Hover over figure to zooma, Antibacterial spectrum of teixobactin. MIC was determined by broth microdilution. aB. anthracis BB resources isolates are from NIH Biodefense and Emerging Infections Research Resources repository. b, Antibacterial activity of teixobactin and known drugs against contemporary clinical isolates. 1In the Viridans Group Streptococci, one isolate of each of the following was tested S. sanguis, S. mitis, S. anginosus, S. intermedius and S. salivarius. PISP, penicillin-intermediate S. pneumoniae; PRSP, penicillin-resistant S. pneumoniae; PSSP, penicillin-sensitive S. pneumoniae.
-
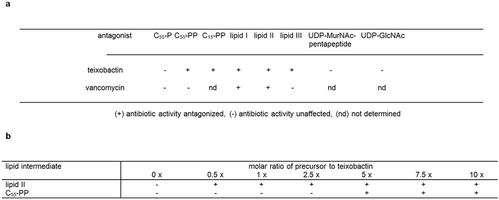
Extended Data Table 2: Antagonization of the antimicrobial activity of teixobactin by cell wall precursors
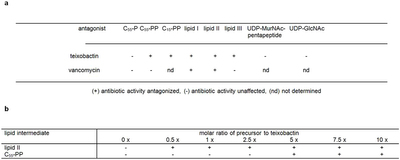 Hover over figure to zoom
Hover over figure to zooma, S. aureus ATCC 29213 was incubated with teixobactin and vancomycin at 8 × MIC in nutrient broth in a microtitre plate, and growth was measured after a 24 h incubation at 37 °C. Putative HPLC-purified antagonists (undecaprenyl-phosphate [C55-P], farnesyl-pyrophosphate [C15-PP], undecaprenyl-pyrophosphate [C55-PP], UDP-MurNAc-pentapeptide, UDP-GlcNAc, lipid I, lipid II, and lipid III) were added in a fivefold molar excess with respect to the antibiotic. b, Teixobactin at 8 × MIC was exposed to increasing concentrations of putative antagonistic lipid intermediates. Experiments were performed with biological replicates.